Biotherapeutic drug development
Biotherapeutic Research Group
Biotherapeutic research group focuses on the development and formulation of novel antibody-based medicine (biotherapeutics) with application in the treatment of chronic inflammatory diseases. This translational research group aims to develop next-generation biotherapeutics with enhanced stability and extended duration of action with great potential for the improvement of human health and well-being. This work will also benefit pharmaceutical companies involved in the biotherapeutic manufacturing and formulation.
Group members
-
Dr Hanieh Khalili
Associate Professor in PharmaceuticsSchool of Medicine and BiosciencesAfter completing my PhD in Pharmaceutics department at University College London (UCL) School of Pharmacy in 2012, I joined the Institute of Ophthalmology at UCL to work as a postdoctoral research associate. During my postdoc, I worked in multidisciplinary translational research group, supervised by Profs Sir Peng Tee Khaw and Steve Brocchini, to develop antibody-based medicine such as bispecific antibodies and fusion proteins to modulate ocular healing after surgery and to develop dosage forms to inhibit inflammation, angiogenesis and fibrosis generally within the eye.
Completed PhD students
- Matthew Collins
Research students
- Camelia Bogdan
- Arian Farokh Boroojerdi
- Wiktoria Roksana Grabowska
- Hamid Heidari Kashkooli
- Sama Pirkalkhoran
Current research themes
- Develop novel biotherapeutics (antibody mimetic, bispecific antibody/peptide, antibody-drug conjugate)
- Pharmaceutical analysis; binding and thermodynamics
- AI application in biopharmaceuticals
- Tissue engineering for retinal degenerative disease; 3D bioprinting
- Engineering of novel antibody fragments
- Biopharmaceutical formulations for pulmonary airway disease
- 3D printing collagen-based scaffold for targeted delivery
Current projects
Developing bispecific antibody mimetics
New classes of protein-based medicines are being investigated with the focus on increasing their functionality and stability. Bispecific IgGs that bind to two different targets simultaneously have been developed mainly in Oncology (blinatumomab and emicizumab).
Recently (2022) the bispecific IgG faricimab has been approved for treatment of age-macular degeneration disease (AMD). This bispecific, however, contains an Fc fragment which may cause immune-related effector functions that can drive (rather than inhibit) inflammation, while also carrying the risk of inadvertent agonism caused by cross-linking with Fc receptor expressing cells.
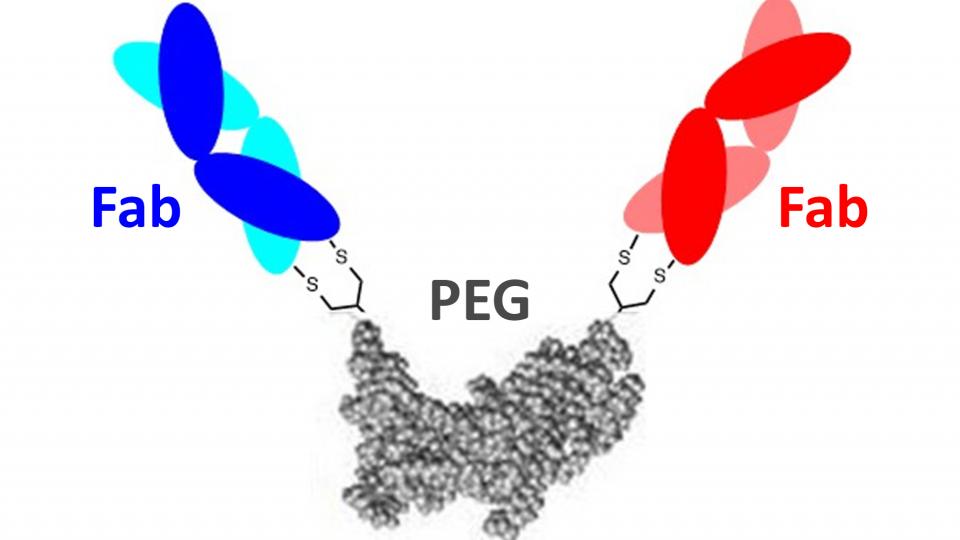
We have previously developed Fc-free antibody mimetic (FpF) targeting to a single target using a chemical-recombinant approach. FpF displayed similar binding and functional actives as IgG.
Given the potential therapeutic advantage of the FpF motif over IgG and an unmet need to develop bispecific IgG for ocular neovascularisation diseases, the main aim of this project is to make bs-FpFs binding two different targets.
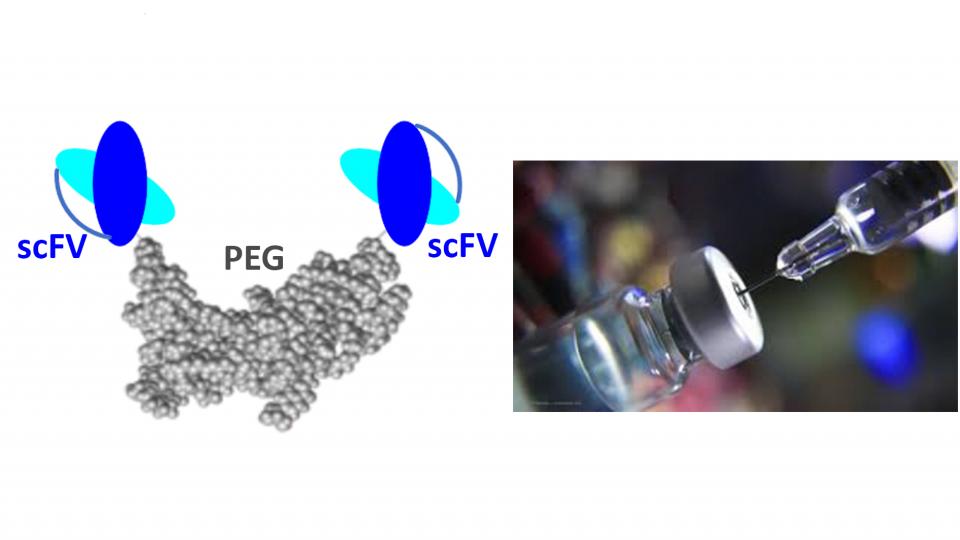
Engineering antibody fragment mimetics (scFv)
Ocular drug delivery of biologics to the posterior segment is an important and rapidly developing field of research because of their applications in treatment of ocular neovascularisation disease such as age-macular degeneration (AMD). Intravitreally administered (IVI) protein-based medicines targeting vascular endothelial growth factor (VEGF), which causes angiogenesis and neovascularisation, have revolutionised the treatment of AMD.
To date, four anti-VEGF antibody-based therapies have been approved for treatment of retinal neovascularisation. However, they require long-term monthly or bimonthly injections, which is difficult for patients, and compliance often decrease after the first year of treatment. There is a need to reduce the frequency of intravitreal injections to treat chronic intraocular disease.
The main aim of this project is to develop long acting ocular biotherapeutics for posterior eye disease to have less frequent of injection and greater retina residence time and exposures. We aim to make antibody fragment mimetics for higher effective dose and improve potency.
Formulating antibody mimetics (FpF)
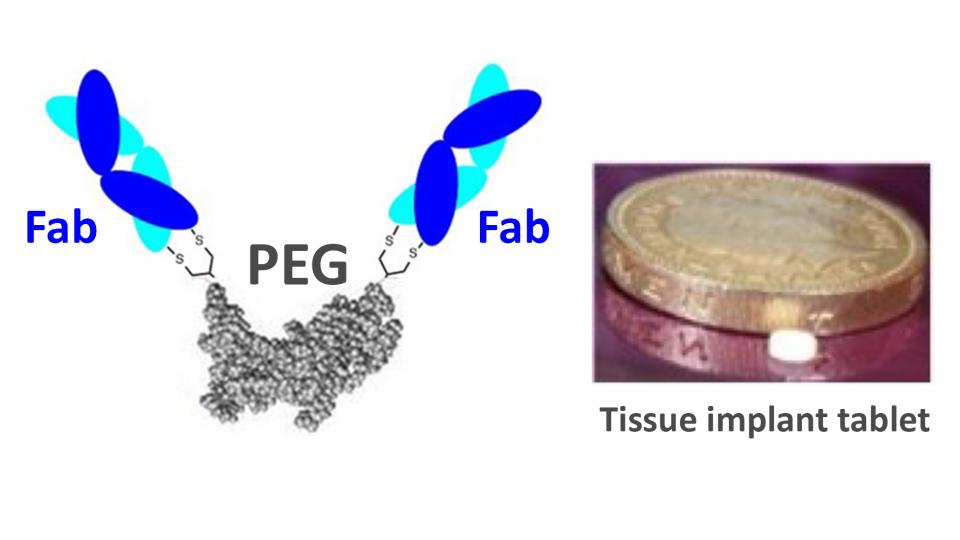
IgG antibodies have some limitations with stability and efficacy. We have developed antibody mimetics (FpF) as a novel IgG mimetic which is designed to be more stable than IgGs. The increased stability of FpFs indicates they can be fabricated into more stable and useful forms than are possible with IgGs.
The main aim of this project is to fabricate and characterise FpFs for the following forms:
- freeze dried forms that do not require a cold chain
- implantable and injectable forms (e.g. small tissue implantable tablets) for tissue specific delivery to decrease dosing frequency and the need for large systemic doses
Creating 3D collagen-based micro-implants for targeted therapy
Retinal impairment diseases including age-related macular degeneration lead to permanent retinal damage and vision loss if left untreated. The replacement of damaged retinal cells is thought to be the best therapeutic strategy.
We have engineered injectable collagen-based materials that we aim to develop for use in 3D printing to fabricate relevant micro-implants. The 3D collagen-based micro-implants will serve as targeted tissue nodes to effectively integrate therapeutic cells into damaged tissues, enhancing the treatment efficacy for vision and nerve impairments.
To achieve this goal, through collaboration with the School of Computing and Engineering's Intelligent Sensing and Vision research group, we will employ artificial neural networks to predict and optimise the material composition and printing process, addressing complex tissue implant patterns and leading to enhanced micro-implant 3D design and production.
PhD projects/topics/interest
We have exciting opportunities for PhD students to join the biotherapeutic research group in the following topics:
-
Engineering of bispecific single chain fragment using chemical-recombinant approach
Supervisors
- Dr Hanieh Khalili (contact detail: hanieh.khalili@uwl.ac.uk)
- Dr David Guiliano (contact detail: david.guiliano@googlemail.com)
Project summary
Single-chain variable fragments (scFvs), in particular bi-specifics (BiTEs) targeting multiple tumour antigens or acting as immune engagers are a key new class of cancer therapeutics. High-yield expression of BiTEs remains a key bottleneck, limiting patient access due to high production costs. This PhD project will tackle this challenge by leveraging our previously developed conjugation-ligation platform to deliver cost-effective and tailorable BiTE production.
This is a collaborative project between the School of Medicine and Bioscience at UWL and the School of Life Sciences at the University of Westminster to investigate the feasibility of engineering single-chain fragment using our established insect cell production system targeting clinically relevant antigens in blood and solid tumours (CD3, CD19, EpCAM).
-
Development of bispecific antibody-based mimetics
Principal supervisor:
Dr Hanieh Khalili
Contact hanieh.khalili@uwl.ac.uk
New classes of protein-based medicines are being investigated with the focus on increasing the functionality and stability. We have so far developed mono-specific antibody-based mimetic which act as IgG mimetics with similar solution size and binding affinity. There is much interest to explore the benefit of using bispecific IgG mimetic in inflammation and fibrosis disease. The mimetic that we have been working on, does not have Fc fragment which its presence may cause immune-related effector functions to drive (rather than inhibit) inflammation.
This PhD proposal is a collaborative work with UCL School of Pharmacy and Institute of Ophthalmology to develop stable, and Fc-free bispecific antibody-based mimetic to be more efficacious than mono specific antibody and even better than simply mixing mono-specific antibodies because of synergistic effect. PhD candidate will work alongside a team of chemist, and pharmacist to develop novel bispecific antibody mimetic and will be trained to work with complex biopharmaceutical formulation techniques and biological assays (protein conjugation, characterisation, purification, protein-protein binding interaction techniques, cell-based in-vitro assays). As part of the project, the student will be given the opportunity to spend a period at UCL School of Pharmacy, to undertake collaborative research using state of art pharmaceutical facilities such as Biacore and ITC.
-
Artificial intelligence aided design to develop 3D scaffold for retinal regeneration
Principal and co-supervisors:
Dr Hanieh Khalili and Prof Massoud Zolgharni
Contact hanieh.khalili@uwl.ac.uk
Retinal tissue engineering is representing a new approach to treat retinal diseases through the development of a biological substitute; the so-called scaffold. A tissue scaffold is a 3-dimentional (3D) structure with interconnected pores which are used to deliver cells, drugs and genes into the local tissue. Scaffold should provide a suitable space in which retinal pigment epithelium (RPE) can grow and generate their own extracellular matrix (ECM). Collagen is the most abundant structural protein that play critical role in maintaining the ECM which has been widely used to fabricate scaffolds for tissue engineering because of its biodegradability, superior biocompatibility and weak antigenicity. Some limitations exist with cross-linked collagens including the use of leachable toxic crosslinkers, a short duration of action, activation of T-cells and difficult injection of the polymerised networked materials. To address these limitations, an in-situ polymerizable collagen (IPC), which is fibrilised without uses of a chemical cross linker, is proposed to use as a scaffold for this PhD proposal.
To fabricate 3D IPC scaffolds with specific shapes and sizes, different methods will be applied such as freeze-drying and 3D bioprinting. Multi-objective optimisations are, however, required to identify suitable freeze-drying and printing conditions and material composition (percentage of cells to IPC) to achieve optimal mechanical and porous constructions properties. This requires extensive experimentation time that is resource demanding. Artificial intelligence (AI) and machine learning (ML) methods have already been applied in tissue engineering and have been shown to be transformative resources to support researchers in the field of regenerative medicine. The ML-based framework takes the material composition and the printing parameters as input to (i) predict printing parameters to optimise the structure’s properties, (ii) assess the quality of the prints and (iii) optimise printability of the material.
This PhD proposal is a collaborative work between the School of Biomedical Science and School of Computing and Engineering to investigate the feasibility of using AI to predict/optimise the physicochemical and mechanical properties of 3D printed IPC cells scaffolds, thereby reducing the time and data needed for experimentations.
Prospective student will have good first degree in Pharmacy, Chemistry or another pharmaceutical-related discipline.
-
Artificial intelligence aided design of 3D printing of immunostimulatory implants for cancer T cell therapy
Principal and co-supervisors
- Dr Hanieh Khalili (contact detail: hanieh.khalili@uwl.ac.uk)
- Dr Karolina Dziemidowicz (contact detail: k.dziemidowicz@ucl.ac.uk)
Project summary
This PhD proposal is a collaborative work between the School of Biomedical Sciences and School of Computing and Engineering at University of West London and School of Pharmacy at University College London (UCL) to investigate the feasibility of using AI to predict/optimise the physicochemical and mechanical properties of 3D printed immunostimulatory scaffolds for cancer T-cells therapy.
T cell therapy is a promising approach in combating certain blood cancers. However, the conventional intravenous delivery of T cells shows limited efficacy against solid tumours. This is primarily attributed to the considerable challenges these cells face in locating, infiltrating and proliferating within the typically immunosuppressive tumour microenvironment following systemic administration.
Implantable biomaterials can potentially enhance T cell therapy outcomes in solid tumours by enabling localised delivery of T cells and immunostimulatory molecules necessary for the maintenance of their function. This PhD project therefore focuses on developing 3D implantable collagen scaffold to facilitate localised delivery of T cell therapy in solid cancers.
Advanced techniques such as 3D bioprinting will be employed to create specific scaffold shapes and sizes. To optimise these scaffolds and ensure T cell viability, various mechanical and physicochemical aspects will be considered. This optimisation will be achieved through machine learning and AI-based framework, which will assess the scaffold properties, evaluate print quality and optimise T cell viability within the 3D printed scaffold. Additionally, the scaffolds will be loaded with microparticles functionalised immunostimulatory biomolecules (cytokines or antibodies) to maintain T cell function and viability in situ.
The interdisciplinary nature of this research involves collaboration with Pharmaceutics, Biomedical Science and Computing and Engineering. The project includes fabricating biomolecule-functionalised microparticles and conducting cellular assays at UCL School of Pharmacy, under supervision of Dr Dziemidowicz. Techniques such as x-ray photoelectron spectroscopy, scanning electron microscopy, cell culture, flow cytometry and confocal microscopy will be used. Additionally, the 3D bioprinting of collagen scaffold will take place in Dr’s Khalili research lab. Finally, machine learning will be designed in Prof Zolgharni’s research group, Intelligent Sensing and Vision (IntSaV).
-
Research degrees with the School of Medicine and Biosciences
Current PhD opportunities in the School of Medicine and Biosciences
Vacancies
- Postdoctoral research fellow in Biopharmaceutics development and formulation
- AI design in biotherapeutics
- Ocular drug delivery
Available by directly contacting hanieh.khalili@uwl.ac.uk.
External partners
-
UCL School of Pharmacy
-
UCL Institute of Ophthalmology
-
University of Westminster
-
University of East London
-
Italian Institute of Technology
-
National Cancer Institute's Center for Cancer Research
Training
The biotherapeutic drug development group has a strong training focus aimed at developing the next generation leaders in the field of biotherapeutics. In addition to its core research activities, the group offers comprehensive PhD and MSc programmes for researchers with a strong biopharmaceutical background. We are training leaders in the highly sought after domains of antibody-based medicine formulation. Our PhD course will enable students to acquire the knowledge on the pharmaceutical techniques, and prepare them for a rewarding career in biopharmaceuticals companies. The programme aims/objectives are to give the students practical experience and the opportunity to acquire competence in up-to-date techniques leading to enhanced professional capabilities.
-
Journal articles
- S. Prikalkhoran, D. Guilian, H. Khalili, Storage Stability and Solution Binding Affinity of an Fc-Fusion Mimetic, Journal of Pharmaceutical Science, Nov 2024. https://doi.org/10.1016/j.xphs.2024.11.016
- M. Collins , N. Ibeanu, WR. Grabowska, S. Awwad, PT. Khaw, S. Brocchini, H. Khalili. Bispecific FpFs: a versatile tool for preclinical antibody development. RSC Chem Biol. 2024 Sep 27. https://doi: 10.1039/d4cb00130c
- Kashkoli, Hamid Heidari, Weyland, David Edward, Pirkalkhoran, Sama and Grabowska, Wiktoria Roksana, Khalili, Hanieh, Advanced therapy medicinal products for age-related macular degeneration; scaffold fabrication and delivery methods. Pharmaceuticals, (2023), 16 (4). p. 620.
- Pirkalkhoran, Sama, Grabowska, Wiktoria Roksana, Kashkoli, Hamid Heidari, Mirhassani, Reihaneh, Guiliano, David, Dolphin, Colin and Khalili, Hanieh, Bioengineering of antibody fragments: challenges and opportunities. Bioengineering, (2023), 10 (2). p. 122.
- M. Collins, H. Khalili, Soluble Papain to Digest Monoclonal Antibodies; Time and Cost-Effective Method to Obtain Fab Fragment” Bioengineering, (2022), 9(5), 209; https://doi.org/10.3390/bioengineering9050209
- M. Collins, S. Awwad, N. Ibeanu, P. T. Khaw, D. Guiliano, S. Brocchini, H. Khalili: “Dual acting therapeutic proteins for intraocular use” Drug Discovery Today, (2021), 26(1):44-55 https://doi.org/10.1016/j.drudis.2020.10.025.
- H.Khalili: “Using different proteolytic enzymes to digest antibody and its impact on stability of antibody mimetics” Journal of Immunological Methods, (2021), 21:112933.
- C. Picken, S. Awwad, M. Zloh, H. Khalili, and S. Brocchini: “Protein Modification by Bis-alkylation” Chapter 16, Polymer-protein Conjugate book, 2020, Pages 351-385
- H. Khalili P. T. Khaw, S. Brocchini, Sergey K. Filippov: “Comparative thermodynamic analysis in solution of a next generation antibody mimetic to VEGF” RSC advance (2018), 8: 35787.
- H. Khalili, R. W. Lee, P. T. Khaw, S. Brocchini, A. Dick, D. Copland: “Anti-TNFa antibody mimetic to treat ocular inflammation” Scientific Reports-Nature. (2016), 6: 36905.
- H. Khalili*, S. Brocchini, P. T. Khaw: “Fc-fusion mimetics”, Biomaterials Science. (2016), 4(6):943‐947. *Corresponding author
- H. Khalili*, S. Brocchini, P. T. Khaw, A. Khalili, G. Sharma: “The increased stability of FpFs compared to monoclonal antibodies”, IOVS, (2015), 56: 377. *Corresponding author
- H. Khalili*, G. Sharma, S. Brocchini, P. T. Khaw: “Storage stability of bevacizumab in polycarbonate and polypropylene syringes”, Nature, Eye (2015), 29: 820-827. *Corresponding author
- C. Ginn, H. Khalili, R. Lever, S. Brocchini: “PEGylation and its impact on the design of new protein-based medicines”, Future Med. Chem (2014), 6(16): 1829–1846.
- H. Khalili, A. Godwin, J. Choi, R. Lever, P. T. Khaw, S. Brocchini: “Fab-PEG-Fab as a potential antibody mimetic” Bioconjugate Chemistry (2013), 24 (11): 1870–1882.
- A. Herrington-Symes, M. Farys, H. Khalili, S. Brocchini: “Antibody fragments: Prolonging circulation half-life special issue-antibody research”, Advances in Bioscience and Biotechnology (2013), 4(5): 689-698.
- M. Farys, C Ginn, G Badescu, K Peciak, E Pawlisz, H. Khalili, S Brocchini: Invited book chapter review on “Chemical and genetic modification” in Biological and Drug Products; development and Strategies book; Wiley 2013, Edited by W. Wang and M. Singh.
- H. Khalili, A. Godwin, J. Choi, R. Lever, S. Brocchini: “Comparative binding of disulfide-bridged PEG-Fabs”, Bioconjugate Chemistry (2012), 23 (11): 2262-77.
- H. Khalili, Rafiee Z, B. Rezaei, Sh. Mallakpour: “A simple and rapid spectrophotometric method for the determination of ultra trace amounts of thallium (III) with 4-(4'-N,N-dimethylaminophenyl) urazole as a new reagent”. Ann Chim. (2005), 95 (11-12): 897-903.
- H. Khalili, B. Rezaei, Sh. Mallakpour: “Spectrophotometric Flow Injection determination of trace amounts of thallium with 4-(4¢-N,N-Dimethylaminophenyl) urazole as a new reagent”. The Canadian Journal of Analytical Sciences and Spectroscopy, (2005), 50 (6).
- H.Khalili: “Dual acting therapeutic” Distinguished speaker at Novel Ophthalmology, USA, 2021.
- H. Khalili: “Antibody mimetic; A better antibody-based medicine” Invited plenary speaker at Pharmaceutical Drug Delivery System, Spain, 2017.
- H. Khalili: “Bispecific cytokine antibody mimetics for vascular disease” Invited speaker in an international workshop on cardiovascular biology and translational medicine, Royal College of Physicians, London, UK, 2016.
- H. Khalili: Invited to run a workshop on “Therapeutic antibodies; Fundamental, Efficacy, and Stability”, AryoGen Pharmed Co. Tehran, Iran, 2016.
- H. Khalili: “Antibody mimetic” Invited speaker in MedImmune KTN formulation meeting, Cambridge, UK, 2015.
- H. Khalili, D. Copland, P. T. Khaw, A. Dick, S. Brocchini: “Ocular inflammation; Infliximab antibody mimetic” Podium in Inflammation and Immunity symposium, London, UK, 2015.
- H. Khalili, et al: “Stability studies of anti-VEGF FpFs obtained from bevacizumab digested with different enzymes” in American Association of Pharmaceutical Scientist (AAPS) Annual Meeting, San Antonio, USA, 2019.
- H. Khalili, et al: “Thermodynamic properties of anti-VEGF F antibody mimetic in the solution” in Control Release Society (CRS) Annual Meeting, Spain, July 2019.
- H. Khalili, et al: “Lyophilisation of an anti-VEGF antibody mimetic to make a solid implant” in Control Release Society (CRS) Annual Meeting, Spain, July 2019.
- H. Khalili, et al: “Binding characterization of bispecific antibody mimetics” in Control Release Society (CRS) Annual Meeting, Spain, July 2019.
Contact us
Dr Hanieh Khalili
- School: School of Medicine and Biosciences
- Address: University of West London, London, W5 5RF, UK
- Phone: 020 8231 2044
- Email: Hanieh.Khalili@uwl.ac.uk
Find out more
-
Research Centres and Groups
Find out about our multi-disciplinary areas of expertise, PhD research, and teaching.
-
Research impact
Learn how our PhD research has helped communities locally, nationally and internationally.
-
The Graduate School
If you are interested in studying for a PhD or Professional Doctorate, the Graduate School is here to support your research.